TNMB classification and clinical staging
Accurate clinical staging of Mycosis Fungoides and Sézary Syndrome is essential for informing prognosis and guiding treatment approach.1-3
Consensus recommendations developed by an international team of CTCL specialists with expertise in Mycosis Fungoides and Sézary Syndrome (ISCL, USCLC, and EORTC) include the TNMB classification system, which can provide the clinical stage (ranging from IA to IVB).4
TNMB classification
To determine a patient’s clinical stage, levels of involvement in 4 compartments are assessed: skin, lymph node, visceral/metastasis, and blood. This is referred to as the TNMB classification.4
TNMB criteria are used to derive clinical stage1
| T SKIN | N LYMPH NODE | M VISCERAL/METASTASIS | B BLOOD |
|---|---|---|---|
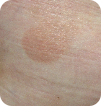
T1 Limited patches/plaques, <10% of skin surface |
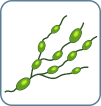
N0 No abnormal nodes |
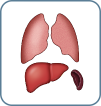
M0 No visceral |
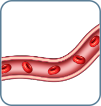
B0 No blood involvement <250 atypical cells/µL |
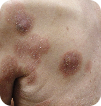
T2 Generalized patches/plaques, ≥10% of skin surface |

N1 Clinically abnormal, histopathology Dutch grade 1 or NCI LN0-2 |
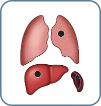
M1 Visceral |
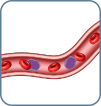
B1 Low-level involvement between 250-1000 atypical cells/µL |
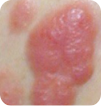
T3 Tumors, 1 or more |

N2 Clinically abnormal, histopathology Dutch grade 2 or NCI LN3 |
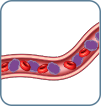
B2 High-level involvement >1000 atypical cells/µL of lymphocytes |
|
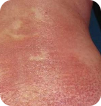
T4 Erythroderma >80% of skin |

N3 Clinically abnormal, Dutch grade 3-4 or NCI LN4 |
Classifications omitted above: T0=absence of clinically significant skin lesions. Nx=clinically abnormal LN but no pathologic determination of representative LN; Mx=unable to determine by pathologic or imaging assessment; Bx=unable to quantify blood involvement according to agreed upon guidelines.
Blood class for each level (B0, B1, B2, Bx) can further be characterized by TCR clone status, with A=clone negative or equivocal and B=clone positive with identical clone in the skin.
Atypical cells are defined as CD4+/CD26- or CD4+/CD7- cells.
Images for T1 and T2 courtesy of Oleg Akilov, MD, PhD. Images for T3 and T4 courtesy of Joan Guitart, MD.
Assessing classification for staging
Skin (T)
Evaluation of the type of lesion(s) and level of skin involvement based on the proportion of body surface area (BSA) affected.4
- In patch/plaque disease, <10% affected BSA indicates T1 classification; ≥10% BSA indicates T2
- The presence of tumors indicates a skin classification of at least T3, regardless of the extent of BSA affected by patches and plaques
- Erythroderma affecting >80% of BSA indicates T4 classification
Evaluating extent of skin involvement
Once CTCL is diagnosed via biopsy, the disease compartments need to be assessed for staging. For skin, this assessment involves identifying and quantifying the type and extent of lesions.4
Guidelines recommend a complete skin examination to assess the percentage of BSA and the type of skin lesion (ie, patch/plaque, tumor, erythroderma). The patient’s palm plus all 5 fingers equals roughly 1% of BSA.
Lymph node (N)
- Palpation of peripheral lymph node regions4
- Imaging is recommended for skin classification ≥T3 or T4 (chest/abdomen/pelvis (C/A/P) with contrast or FDG-PET CT)
- Imaging may be useful for ≥T2b, or with palpable adenopathy or abnormal laboratory studies
- Imaging may be considered for ≥T2a if clinically indicated
- Lymph node biopsy for staging is recommended for clinically abnormal nodes (>1.5 cm in longest diameter)4
- Consultation with CTCL specialist is recommended for suspected lymph node involvement3
Viscera (M)4
- Palpation for organomegaly/masses
- Imaging is recommended for suspected visceral involvement (C/A/P CT with contrast or integrated whole body FDG-PET CT)
Blood (B)
- Flow cytometry is the preferred method for evaluating blood. Sézary cell preparation is considered more subjective and therefore less useful than flow cytometry2,4
Evaluation of the type of lesion(s) and level of skin involvement based on the proportion of body surface area (BSA) affected.4
- In patch/plaque disease, <10% affected BSA indicates T1 classification; ≥10% BSA indicates T2
- The presence of tumors indicates a skin classification of at least T3, regardless of the extent of BSA affected by patches and plaques
- Erythroderma affecting >80% of BSA indicates T4 classification
Evaluating extent of skin involvement
Once CTCL is diagnosed via biopsy, the disease compartments need to be assessed for staging. For skin, this assessment involves identifying and quantifying the type and extent of lesions.4
Guidelines recommend a complete skin examination to assess the percentage of BSA and the type of skin lesion (ie, patch/plaque, tumor, erythroderma). The patient’s palm plus all 5 fingers equals roughly 1% of BSA.
- Palpation of peripheral lymph node regions4
- Imaging is recommended for skin classification ≥T3 or T4 (chest/abdomen/pelvis (C/A/P) with contrast or FDG-PET CT)
- Imaging may be useful for ≥T2b, or with palpable adenopathy or abnormal laboratory studies
- Imaging may be considered for ≥T2a if clinically indicated
- Lymph node biopsy for staging is recommended for clinically abnormal nodes (>1.5 cm in longest diameter)4
- Consultation with CTCL specialist is recommended for suspected lymph node involvement3
- Palpation for organomegaly/
masses - Imaging is recommended for suspected visceral involvement (C/A/P CT with contrast or integrated whole body FDG-PET CT)
- Flow cytometry is the preferred method for evaluating blood. Sézary cell preparation is considered more subjective and therefore less useful than flow cytometry2,4

Clinical staging
After TNMB classifications are assessed, clinical stage can be determined based on the chart below.
| TNMB classification for Mycosis Fungoides/ Sézary Syndrome6 |
|||||
|---|---|---|---|---|---|
| Clinical stage | Classification | ||||
| T | N | M | B | ||
| Early Stage |
IA |
1 |
0 |
0 |
0,1 |
IB |
2 |
0 |
0 |
0,1 |
|
IIA |
1,2 |
1,2 |
0 |
0,1 |
|
| Advanced Stage |
IIB |
3 |
0-2 |
0 |
0,1 |
IIIA |
4 |
0-2 |
0 |
0 |
|
IIIB |
4 |
0-2 |
0 |
1 |
|
IVA1 |
1-4 |
0-2 |
0 |
2 |
|
IVA2 |
1-4 |
3 |
0 |
0-2 |
|
IVB |
1-4 |
0-3 |
1 |
0-2 |
|
1 in 5 patients with
early-stage (IA-IIA) Mycosis
Fungoides have B1
blood involvement. Blood involvement can occur at early stages of MF.3,7
Based on a PROCLIPI database study that analyzed hematological data from 348 patients with early-stage Mycosis Fungoides.7

Negative prognostic factors
Staging and baseline assessment can help identify patients at risk of progression to more advanced stages.8-11
Patients are more likely to progress if they are diagnosed at a higher stage, have T3 (tumor) skin involvement, low-level blood involvement (as measured by TCR or flow cytometry), and higher lactate dehydrogenase (LDH) levels.8,11 Factors that contribute to a negative prognosis include8,9:
- Age >60 years
- Advanced stage disease
- Presence of extracutaneous disease
- Higher T-stage
- Identical T-cell clone in the skin and in the blood
- Folliculotropism
- Higher level of blood involvement
- Lymph node enlargement
- Eosinophilia and lymph node involvement >N1/Nx
- Elevated LDH level
- Large-cell transformation (defined histologically as >25% of tumor cells displaying large size)
Black patients generally face a worse prognosis compared with non-Black patients.12,13
- Diagnosed at younger ages and more advanced stages
- Younger Black patients (<60 years; excluding those with hypopigmentation) experience more aggressive disease, regardless of stage14
Disease progression
Progression can manifest in a range of possible signs, including:
- Waning response to treatment2
- Visible changes in skin15
- Patients who are in remission may relapse with a mixture of lesion types15
- Worsening skin symptoms (eg, pruritus, chronic and recurring infection, burning pain or sharp “pins and needles” sensation)16-18
- Signs of extracutaneous disease (eg, lymph node enlargement)8
It’s important to stay vigilant and monitor for signs of progression throughout the disease course.
Up to 1 in 3 patients
with Mycosis
Fungoides
may experience disease
progression within
skin or to other compartments,
including blood1,a
aBased on a survival outcomes and prognostic factors study that included 1502 patients with Mycosis Fungoides/Sézary Syndrome.1

Survival by stage
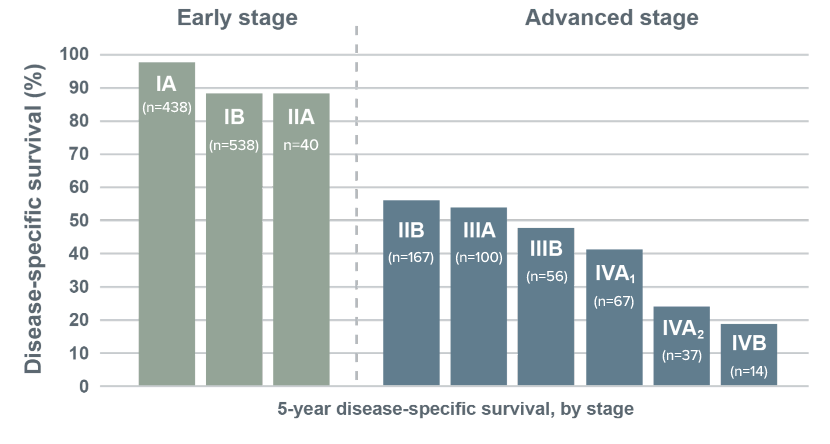
For patients with Mycosis Fungoides or Sézary Syndrome, 5-year disease-specific survival has been shown to drop considerably in advanced stages.1
Shorter disease-specific survival at higher stages1

References: 1. Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28(31):4730-4739. 2. Vermeer MH, Moins-Teisserenc H, Bagot M, et al. Flow cytometry for the assessment of blood tumour burden in cutaneous T-cell lymphoma: towards a standardized approach. Br J Dermatol. 2022;187(1):21-28. 3. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Primary Cutaneous Lymphomas V2.2025. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed September 6, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 4. Olsen EA, Whittaker S, Willemeze R, et al. Primary cutaneous lymphoma: recommendations for clinical trial design and staging update from the ISCL, USCLC, and EORTC. Blood. 2022;140(5):419-437. 5. Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:1713-1722. 6. Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sezary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Forse of the European Organisation for Research and Treatment Center. J Clin Oncol. 2011;29:2598-2607. 7. Scarisbrick JJ, Quaglino P, Prince HM, et al. The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br J Dermatol. 2019;181(2):231-232. 8. Allen PB, McCook AA, Switchenko JM, et al. Staging lymph nodes and blood at diagnosis in mycosis fungoides identifies patients at increased risk of progression to advanced stage: A retrospective cohort study. Cancer. 2023;129:541-550. 9. Farabi B, Seminario-Vidal L, Jamgochian M, et al. Updated review on prognostic factors in mycosis fungoides and new skin lymphoma trials. J Cosmet Dermatol. 2021;21(7):2742-2748. 10. Allen P, McCook AA, Switchenko JM, et al. Risk factors for progression from early to advanced stage mycosis fungoides: a single center retrospective-analysis at the Winship Cancer Institute of Emory University. Blood. 2021;138:1378-1380. 11. Quaglino P, Pimpinelli N, Berti E, et al. Time course, clinical pathways, and long-term hazards risk trends of disease progression in patients with classic mycosis fungoides. Cancer. 2012;118:5830-5839. 12. Nath SK, Yu JB, Wilson LD. Poorer prognosis of African-Americans with mycosis fungoides: an analysis of the SEER dataset, 1988–2008. Clin Lymphoma Myeloma Leuk. 2014;14(5):419-423. 13. Bradford PT, Devesa SS, Anderson WF, Toro JR. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113:5064-5073. 14. Allen PA, Goyal S, Niyogusaba T, et al. Clinical presentation and outcome differences between black patients and patients of other races and ethnicities with mycosis fungoides and Sezary syndrome. JAMA Dermatol. 2022;158(11):1293-1299. 15. Cerroni L. Mycosis fungoides—clinical and histopathologic features, differential diagnosis, and treatment. Semin Cutan Med Surg. 2018;37(1):2-10. 16. Serrano L, Martinez-Escala ME, Zhou XA, Guitart J. Pruritus in cutaneous T-cell lymphoma and its management. Dermatol Clin. 2018;36(3):245-258. 17. Dummer R, Vermeer MH, Scarisbrick JJ, et al. Cutaneous T cell lymphoma. Nat Rev Dis Primers. 2021;7(1):61. 18. Field H, Gao L, Motwani P, Wong HK. Pruritus reduction with systemic anti-lymphoma treatments in patients with cutaneous T cell lymphoma: a narrative review. Dermatol Ther (Heidelb). 2016;6(4):579-595.